Water to Steam
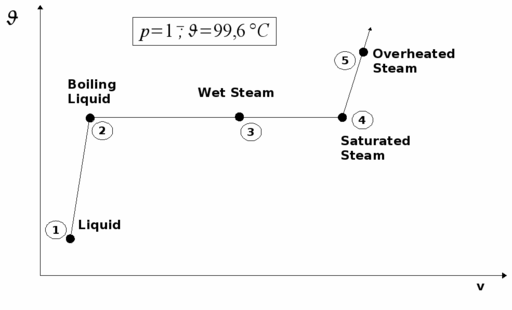
We are looking at the evaporation of water at 1 bar constant pressure.
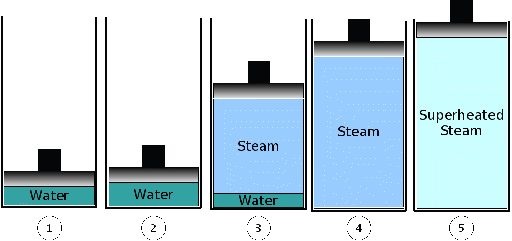
At ambient temperature we find water in liquid phase. When we heat it up, its temperature will rise and the specific volume expands.
At state two, at 99.6 °C, the first bubbles arise. We reach the boiling point. With more heat supply, more steam will arise, the volume expands, but the temperature remains constant (state 3). In this state water and steam exist side by side. This area is called wet steam area.
Finally, at state 4, the last water drop vaporizes and we reach the saturation steam pont.
The alteration of temperature and specific volume during this process is shown by a ϑ/v diagram: